About
Understanding the mechanisms of T lymphocyte tolerance in order to develop innovative strategies to prevent and treat autoimmune pathologies.
The immune system has the remarkable ability to discriminate between self and non-self. The absence or control of immune responses to our tissues is called "immunological tolerance." This essential process prevents potential attacks by immune cells against our tissues. Dysregulation leads to the appearance of autoimmune pathologies that affect 5 to 10% of the population. The thymus plays a vital role in establishing tolerance of T lymphocytes, major players in the immune system due to their ability to fight infections and tumors.
Magali Irla and her team aim to characterize the cellular players in immune tolerance and understand their molecular mechanisms in order to identify new therapeutic strategies for autoimmune diseases and immune system regeneration.
The thymus, a primary lymphoid organ, coordinates developmental processes leading to the generation of mature, functional, non-self-reactive T lymphocytes. The establishment of T cell tolerance in this organ is characterized by two major processes:
– The elimination of autoreactive T cells that recognize self-antigens. This process is called clonal deletion or negative selection.
– Generation of Foxp3 regulatory T lymphocytes (Treg)+ which possess the unique ability to control autoreactive T cells that have escaped thymic selection. Tregs thus play an essential role in maintaining tolerance in the periphery.
Both processes take place primarily in the thymic medulla, which is composed of a dense network of thymic medullary epithelial cells (mTECs) and dendritic cells (DCs). mTECs play a key role in T cell selection due to their fascinating ability to express thousands of peripheral tissue-specific antigens (called autoantigens).
DCs also participate in the establishment of T cell tolerance by presenting self-antigens expressed by mTECs and by transporting harmless antigens captured in the periphery into the thymus. Thus, mTECs and DCs collaborate closely to generate functional non-autoreactive T cells and Treg cells.
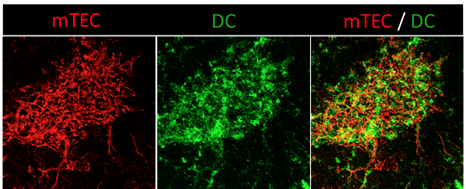
The thymic medulla, the site of T cell tolerance, is composed of a dense network of mTECs and DCs. Confocal microscopy image of a thymus section showing mTECs (anti-keratin 14, red) and DCs (anti-CD11c, green). Copyright: Magali Irla, CIML.
Conversely, developing T cells, called thymocytes, control the differentiation and organization of mTECs. This bidirectional exchange phenomenon is called "thymic crosstalk." In this context, the team showed that CD4 thymocytes+ play a key role not only in the development of functional mTECs expressing the transcription factor Aire but also in the 3D organization of the thymic medulla.
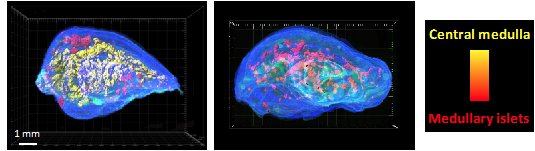
CD4 thymocytes+ control the 3D organization of the thymic medulla. 3D reconstruction of thymic lobes from wild-type mice (left image) and H2A-a mice-/- deficient in CD4 thymocytes+ (right image) using our For3D algorithm (Full organ reconstruction in 3D). Medullary islets are represented by color according to their volume, from red (smallest) to yellow (largest). Copyright: Irla et al. Journal of Immunology 2013.
Furthermore, Treg cells hold great promise in cell therapy for treating autoimmune diseases and inducing tolerance in transplantation. We are therefore studying their development in the thymus and seeking to identify molecules that control their suppressive and anti-inflammatory activity to facilitate their clinical use. To this end, we are using several preclinical mouse models of autoimmunity and transplantation.
Regeneration of function thymic
Thymic function is impaired by myeloablative therapies commonly used to treat hematological diseases through hematopoietic stem cell transplantation. Furthermore, during aging, this organ undergoes progressive changes in its architecture and composition, a phenomenon called "thymic involution." Myeloablative treatments and aging have deleterious effects on TECs, leading to a greatly reduced production of T lymphocytes and consequently to an increased susceptibility to opportunistic infections, autoimmunity, and the development of cancer.
It is therefore essential to identify molecules capable of regenerating the thymus, an organ exhibiting great plasticity. Our team seeks to define new therapeutic strategies to stimulate thymus regeneration and T cell production in order to improve T immunity in numerous physiopathological conditions.
T-cell acute lymphoblastic leukemia (T-ALL)
T-cell acute lymphoblastic leukemia (T-ALL) is a highly aggressive form of leukemia resulting from the malignant transformation of T lymphocytes in the thymus. Despite current treatments, approximately 20% of pediatric T-ALL patients and 50% of adult T-ALL patients do not achieve sustained remission and die from disease progression. We are investigating innovative therapies to prevent T-ALL progression.
News

Suite à l’annonce du Prix Nobel de Médecine 2025, Magali Irla, Directrice de Recherche Inserm, chef d’équipe au Centre d’Immunologie […]

Le week-end du 11 et 12 octobre, Magali Irla et Romain Trubert ont brillamment représenté le CIML au Village des […]
Projects
Project: Reality Foxp3+ regulatory T cells (Tregs) are crucial players in the control of autoimmune and inflammatory diseases […]

Project: SelfExpress The thymus plays a critical role in establishing central tolerance by producing a diverse repertoire […]

Project: ThymMap T lymphocytes recognize pathogens while being tolerant to our tissues. They differentiate […]

Project: T-ALL Leukemias T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive blood cancer, representing 25% of […]

Acronym: TollSOME Obesity is a global health challenge linked to diabetes, hypertension, and cardiovascular disease. While it often stems from […]










