Members
















About
Understanding B cell plasticity: from physiology to pathologies
The “Integrative B Cell Immunology” team studies the fundamental mechanisms that govern humoral immune responses in normal and pathological conditions. B lymphocytes are an essential pillar of our adaptive immune system, responsible for the production of neutralizing antibodies that protect us against infections and participate in immunological memory. Their dysfunction can lead to serious pathologies: malignant transformation in B lymphomas, inadequate responses in cancer, autoimmune diseases or immune deficiencies. Our integrative approach combines innovative methodological developments, fundamental discoveries and translational applications to decipher how these cells adapt to immunological challenges in physiological and pathological conditions.
Technological innovation in the service of immunology
B lymphocytes have a unique characteristic: each cell expresses a distinct antigen receptor, generating a potential diversity of 1013 different specificities. This massive heterogeneity requires sophisticated analytical approaches to understand how the body selects and amplifies B lymphocytes expressing the “right” antigen receptors. Our FB5P-seq platform, developed and patented by the team, allows for the simultaneous analysis of the molecular identity, functional state, and antigenic specificity of individual cells. This technological innovation addresses a critical need in the scientific community: understanding cellular heterogeneity in its natural complexity rather than through averages that mask biological diversity.
Germinal centers: natural laboratories of molecular evolution
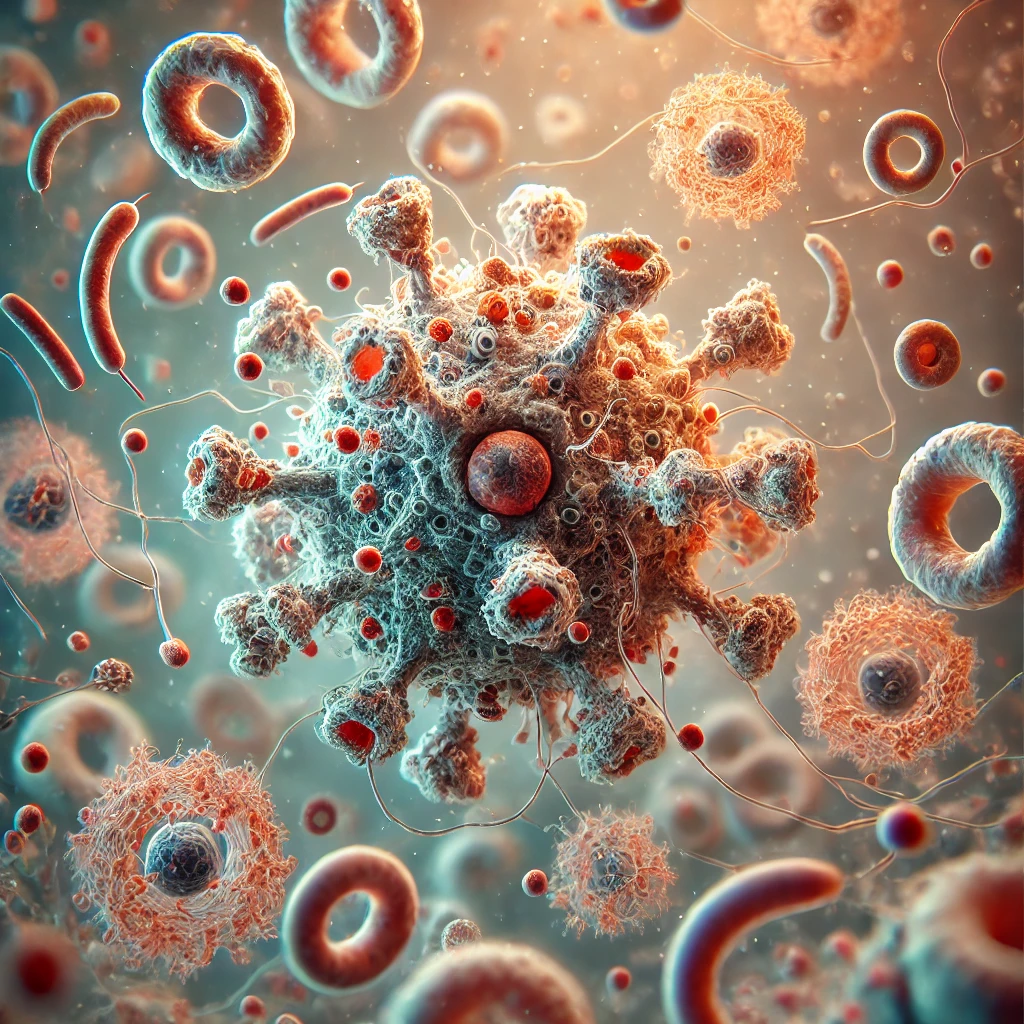
At the heart of our immune system are germinal centers, microscopic structures where a true “Darwinian evolution” of B lymphocytes takes place. These sites are crucial for expanding the diversity of antigen receptors and thus generating high-affinity antibodies during infections or vaccinations. Their dysfunction contributes to vaccine ineffectiveness and autoimmune diseases, or can lead to the development of cancers. Our research aims to understand the rules of this natural selection: what criteria determine that a B lymphocyte will be selected to become a protective memory cell? How do molecular interactions between B lymphocytes and their environment influence selection? These fundamental questions have direct implications for improving vaccine strategies and understanding the mechanisms involved in the development of certain autoimmune diseases and cancers.
Lymphomas: when cancer cells retain their plasticity
Lymphomas represent a heterogeneous group of cancers in which lymphocytes that have become tumors often bear traces of immune activation and one or more passages through germinal centers. By studying cancer cells from different types of lymphomas using our high-resolution molecular tools, our work in the field of hemato-oncology demonstrates that these cells retain remarkable functional plasticity. This discovery revolutionizes our understanding of these diseases and opens up new therapeutic perspectives. By characterizing the recurrent cellular “archetypes” of cancer cells and determining whether their proportions are associated with response to treatments, our work will contribute to the development of personalized medicine for patients with lymphomas.
Anti-tumor immunity: decoding B responses in cancer
B lymphocyte infiltration of solid tumors represents a paradox in immuno-oncology: can these cells contribute to anti-tumor immunity or do they facilitate tumor progression? Our research on lung cancer, the leading cause of cancer mortality worldwide, reveals that tumor-infiltrating B lymphocytes develop antibody responses directed against intracellular autoantigens, suggesting immune recognition of tumor danger signals. This observation could explain why the presence of B-cell-rich tertiary lymphoid structures is often associated with a better prognosis. More importantly, these local autoantibody responses leave a detectable imprint in the bloodstream, opening the possibility of developing non-invasive biomarkers for early cancer diagnosis. This approach, which we are pursuing through collaborative projects involving physicians, researchers, and industry, could transform lung cancer detection, in a context where early detection would significantly improve patient prognosis.
Role of Peyer's patch phagocytes in the initiation of the intestinal immune response
Research group led by Dr. Hugues Lelouard, Research Director at the CNRS.
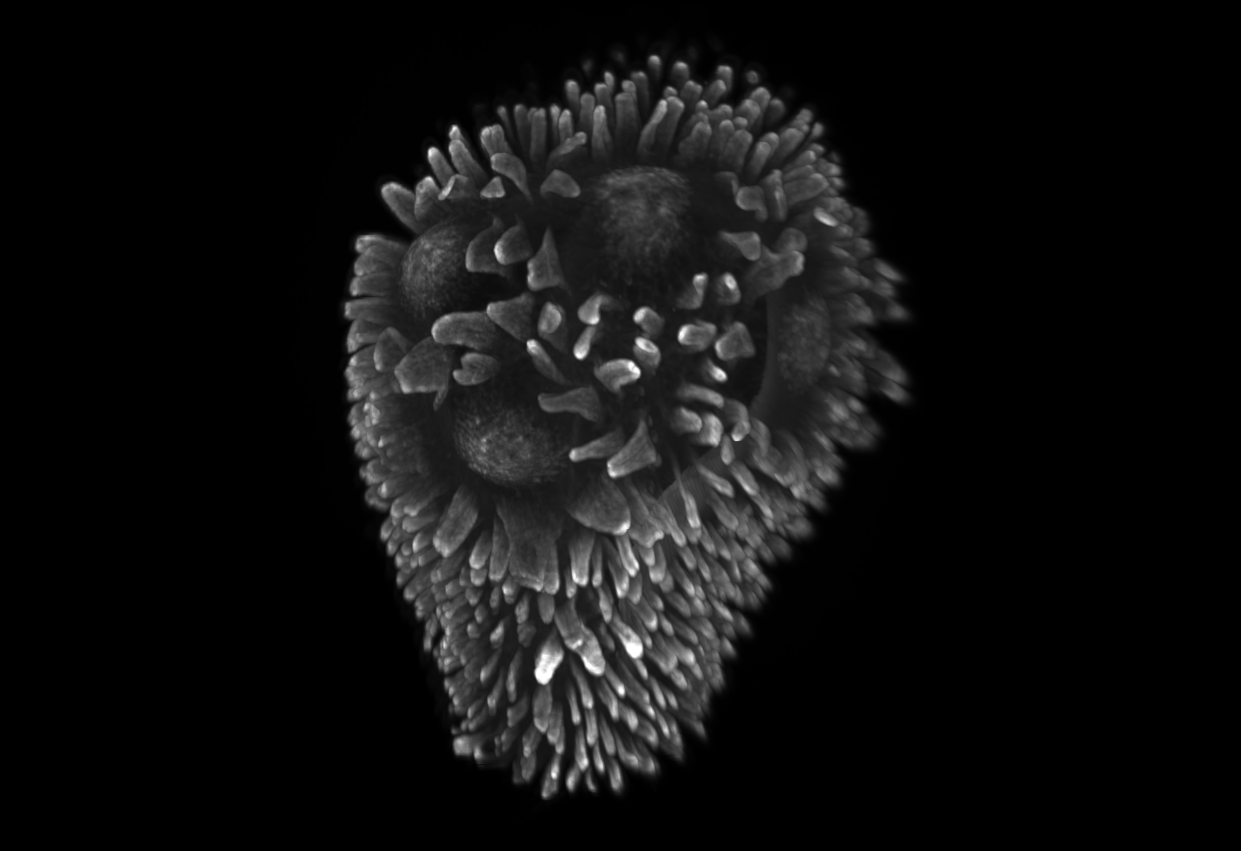
Peyer's patches distributed along the mammalian small intestine are major sentinel and inducer sites of intestinal immunity. Formed by multiple domed B-cell follicles on the mucosal surface, they play a central role in the generation of antigen-specific IgA-secreting plasma cells, which then colonize the lamina propria of the villi. Their ability to discriminate pathogens from the vast majority of harmless antigens from the diet and microbiota, and to trigger an adapted immune response, relies on a complex phagocyte network comprising various macrophage and dendritic cell (DC) subtypes. After performing a thorough characterization of this phagocytic system, we investigated some of its key functions (antigen capture, innate defense, and antigen presentation). Our work has notably highlighted a new population of monocyte-derived dendritic cells, LysoDCs, characterized by a high expression of the antibacterial agent lysozyme and by an increased capacity to capture enteropathogenic bacteria. Absent from intestinal villi, LysoDCs are mainly localized in the subepithelial region of Peyer's patches, where they project dendrites through transcellular pores specifically formed by M cells to directly sample the contents of the intestinal lumen. Their ability to capture antigens from the intestinal lumen but also to initiate a helper and cytotoxic T lymphocyte response makes LysoDCs a prime target for oral vaccination. More recently, we have elucidated the differentiation, maturation and adjuvant activation pathways of LysoDCs, as well as other dendritic cell subpopulations. Our current research focuses on the identification of blood precursors and trophic factors regulating the survival and differentiation of LysoDCs (LysoDiff Project, funded by the ANR), as well as on the establishment of intestinal immunity in Peyer's patches during the weaning period.
Translational impact and future prospects
Beyond addressing fundamental questions about immune system maturation and function, the team's integrative approach, supported by diverse funding including academic and industrial partnerships, aims to transform fundamental discoveries into tangible clinical benefits. Our participation in University Hospital Research projects illustrates this translational approach, where tools developed in the laboratory are directly applied to patient cohorts to identify new diagnostic and prognostic biomarkers. This strategy is part of a broader vision of precision medicine, where the detailed characterization of individual immune responses will help optimize treatments. Collaborating closely with the CIML's technological platforms, we also contribute to the influence of these approaches at the regional and national levels, participating in the emergence of an integrative immunology research ecosystem capable of meeting contemporary human health challenges.
News
Inserm announces funding of the ANTIBAX project to the tune of 2.3 million euros as part of the Health Impact program […]

Le chercheur marseillais est récompensé pour ses travaux sur l’immunologie des lymphocytes B Marseille, le 2 décembre 2025 – Le […]
Projects
Our health depends on a delicate balance between immune defense against pathogens and tolerance to […]


Project: LYMPHOMATLAS Single-cell multi-omic studies in B-lymphomas have highlighted a […]


Project: DECITIP Plasmacytoid dendritic cells (pDCs) excel in the production of type I and III interferons (IFN), […]


Project: ICARO Current acellular vaccines are mostly effective against extracellular pathogens, but fail to induce a response […]


Project: PPTuft This project coordinated by Philippe Jay (IGF, Montpellier) in partnership with our team aims to determine the […]

Patients with follicular lymphoma (FL) present a very high biological heterogeneity, which strongly impacts the response to current therapies. […]

Project: PIONeeR Lung cancer is the leading cause of cancer death, with more than 1.5 million […]


The ANTIBAX project, funded by the France 2030 “Health Impact” program, aims to develop therapeutic antibodies targeting bacteria […]

The TCELL-CODE project, coordinated by Dr. Rémy Lasserre at the Marseille Cancer Research Center, aims to elucidate […]


The PLAIR project, coordinated by Dr Amédée Renand (CR2TI, Nantes), aims to identify the molecular pathways controlling the response […]

The structuring action Spatial Omics of the CALYM consortium, coordinated by Dr Pierre Milpied, aims to develop a virtual platform dedicated […]

The University Hospital Research (RHU) project LUCA-pi (Lung Cancer prevention and interception), coordinated by Professor David Boulate (AP-HM), aims […]

The GCselection project, coordinated by Dr. Pierre Milpied (CIML), revisits the mechanisms of antibody maturation in centers […]


The ST-omics structuring action of the Cancéropôle de la Région Sud is co-coordinated by Dr Pierre Milpied (CIML), Prof Emmanuelle […]

The AITLas-Impact project, coordinated by Pierre Milpied (CIML) in collaboration with Professor François Lemonnier (Mondor Institute of Biomedical Research, […]

Dr. Pierre Milpied's team at CIML is participating in a collaborative project coordinated by Dr. Jean-Pierre de Villartay (Institut […]