About
Our Lab’s Research Focus
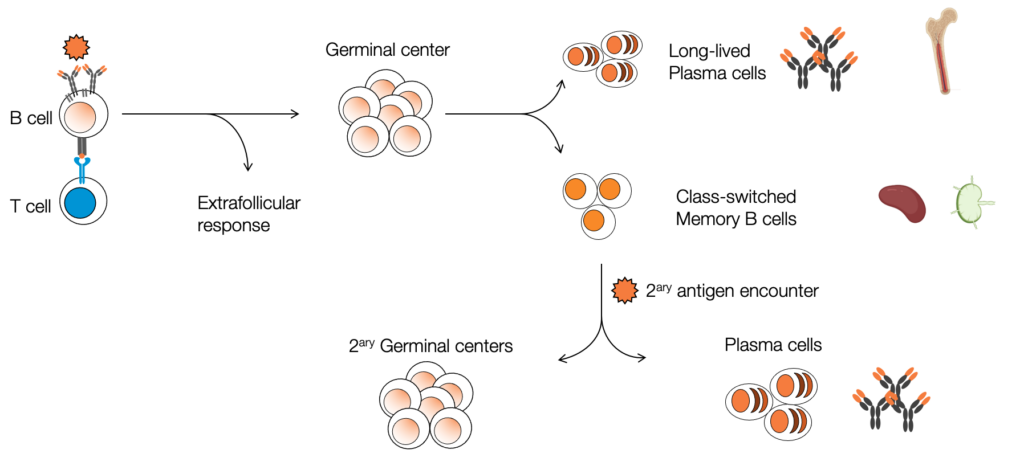
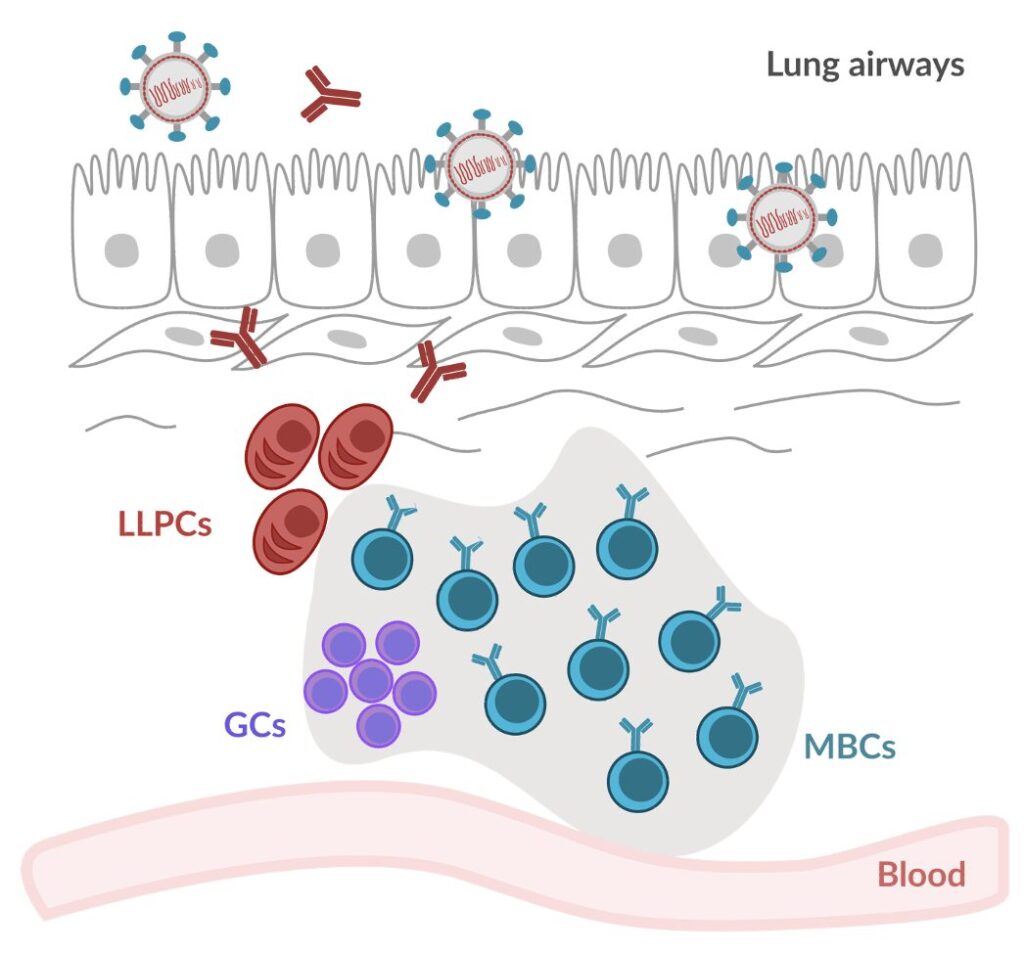
Our lab investigates how the immune system establishes long-lasting protection after infection, with a focus on B cell memory. During an immune response, B cells undergo activation and selection processes that lead to the generation of two key memory populations:
- Long-lived plasma cells (LLPCs): These cells migrate to the bone marrow, where they continuously secrete high-affinity antibodies, providing durable protection that can last for years or even a lifetime.
- Memory B cells (MBCs): These cells remain in a quiescent state in secondary lymphoid organs, such as the spleen and lymph nodes, until they encounter the same pathogen—or a variant—again. Upon reactivation, MBCs can rapidly differentiate into antibody-secreting plasma cells or re-enter germinal centers to further diversify the immune repertoire.
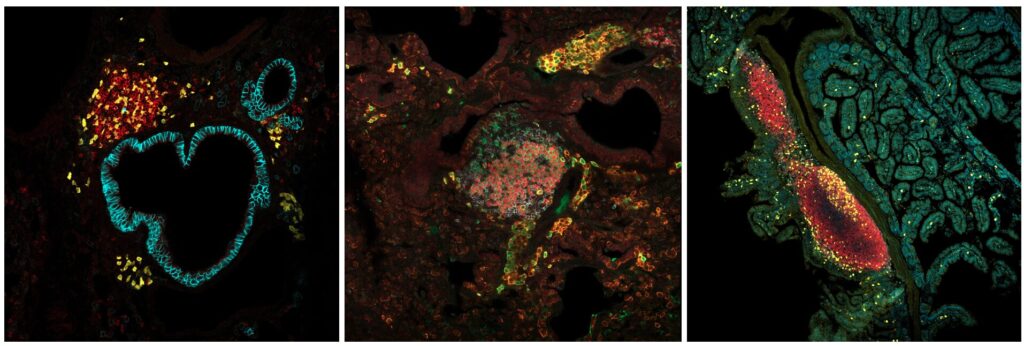
Traditionally, B cell memory has been studied in secondary lymphoid organs. However, recent work from our group and others has shown that LLPCs and MBCs can also take up residence in barrier tissues, such as the lungs, following mucosal infections like influenza and SARS-CoV-2. These populations appear to originate directly from germinal center reactions occurring in tertiary lymphoid structures within the tissue itself. Interestingly, the mechanisms governing germinal center selection and memory formation in barrier tissues seem to differ from those in classical lymphoid organs.
Understanding how B cell memory is generated and maintained in these tissues is essential for several reasons:
- Vaccine development: Many next-generation vaccines, including intranasal and oral formulations, aim to induce mucosal immunity. Defining how B cell responses are triggered directly in barrier sites will help optimize these strategies.
- Immune regulation in disease: While tissue-resident B cell populations can be protective during infection, they may also contribute to chronic inflammation or pathology, such as in allergic asthma.
Our research aims to uncover the signals and cellular interactions that drive germinal center reactions and memory B cell differentiation in barrier tissues like the lung and gut. By doing so, we hope to advance the development of vaccines and therapies that harness mucosal immunity while avoiding harmful immune responses.