Publication: Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors.
Published in: Immunity 2005 May; 22(5): 551-60
Authors: Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, Maina V, Magistrelli G, Haeuw JF, Hoeffel G, Thieblemont N, Corvaia N, Garlanda C, Delneste Y, Mantovani A
Summary
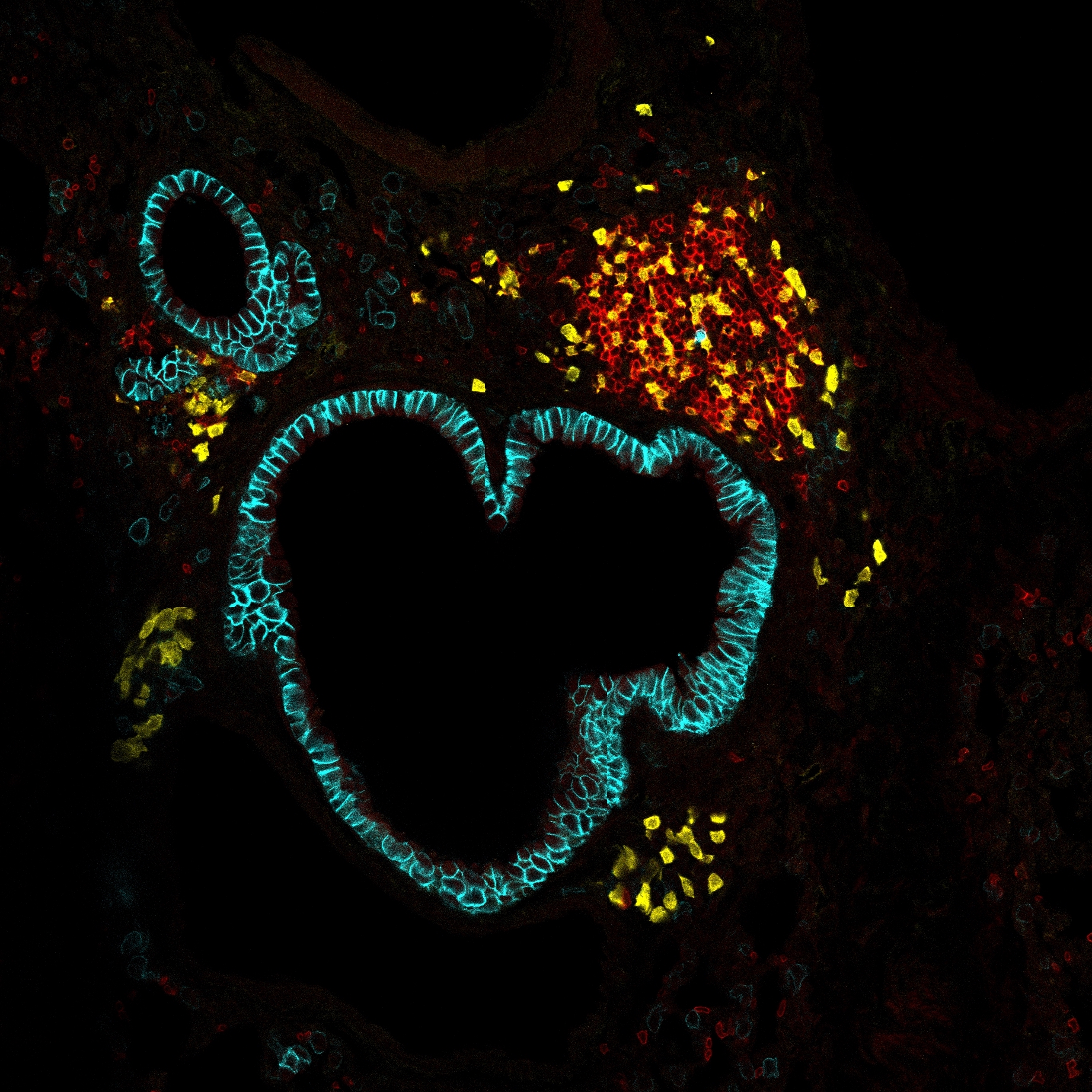
Outer membrane protein A (OmpA) is a conserved major component of the outer membrane of Enterobacteriaceae. Here, we report that OmpA from Klebsiella pneumoniae (KpOmpA) activates macrophages and dendritic cells (DCs) in a TLR2-dependent way. However, TLR2 does not account for binding of KpOmpA to innate immune cells. KpOmpA binds the scavenger receptors (SRs) LOX-1 and SREC-I, but not other members of the same family. LOX-1 colocalizes and cooperates with TLR2 in triggering cellular responses. The TLR2-activated functional program includes production of the long pentraxin PTX3, a soluble pattern recognition receptor involved in resistance against diverse pathogens. PTX3, in turn, binds KpOmpA but does not affect recognition of this microbial moiety by cellular receptors. KpOmpA-elicited in vivo inflammation is abrogated in TLR2(-/-) mice and significantly reduced in PTX3(-/-) mice. Thus, SR-mediated KpOmpA recognition and TLR2-dependent cellular activation set in motion a nonredundant PTX3-mediated humoral amplification loop of innate immunity.
Link to Pubmed [PMID] – 15894273