Publication: NF-κB-dependent IRF1 activation programs cDC1 dendritic cells to drive antitumor immunity.
Published in: Sci Immunol 2021 Jul; 6(61):
Authors: Ghislat G, Cheema AS, Baudoin E, Verthuy C, Ballester PJ, Crozat K, Attaf N, Dong C, Milpied P, Malissen B, Auphan-Anezin N, Manh TPV, Dalod M, Lawrence T
Summary
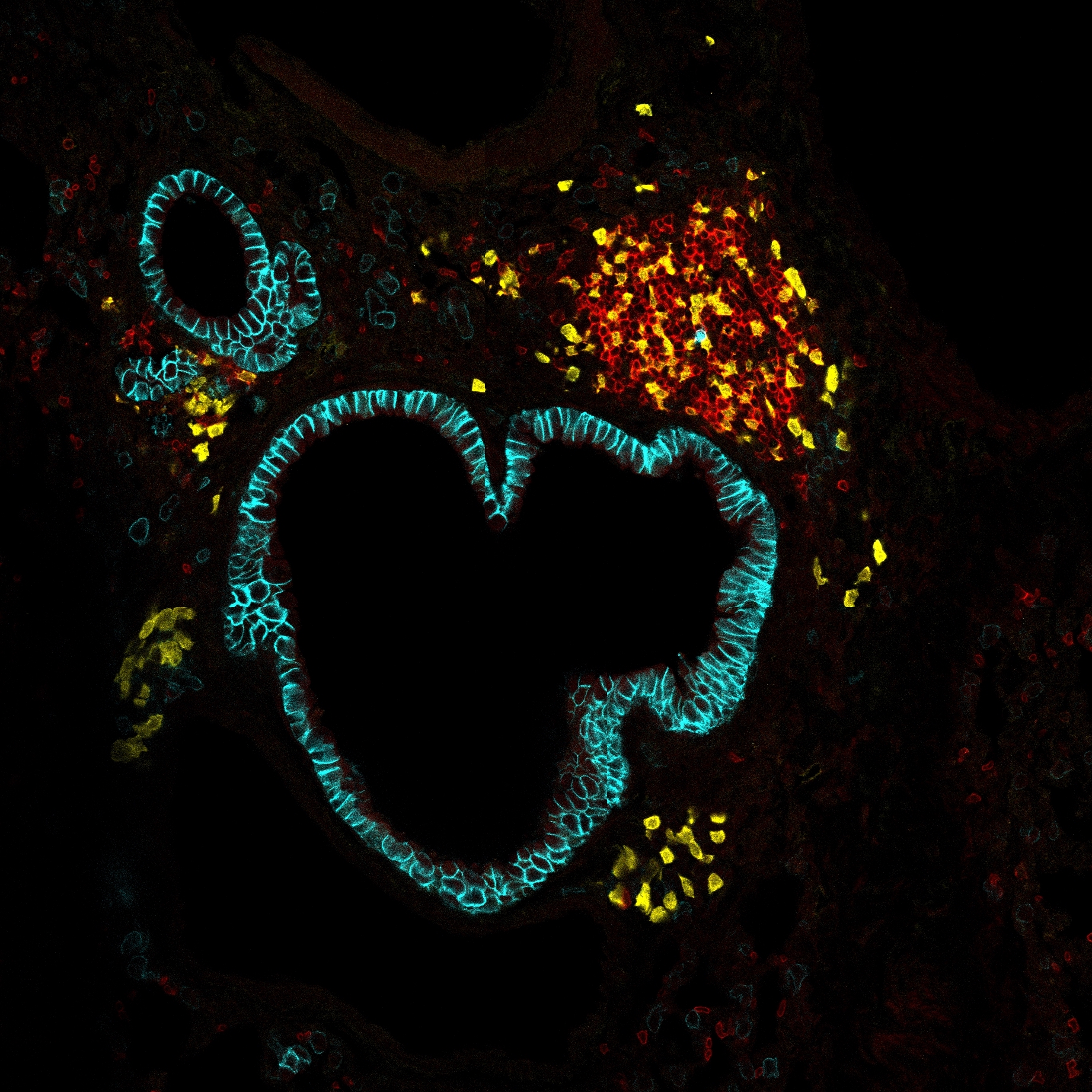
Conventional type 1 dendritic cells (cDC1s) are critical for antitumor immunity. They acquire antigens from dying tumor cells and cross-present them to CD8+ T cells, promoting the expansion of tumor-specific cytotoxic T cells. However, the signaling pathways that govern the antitumor functions of cDC1s in immunogenic tumors are poorly understood. Using single-cell transcriptomics to examine the molecular pathways regulating intratumoral cDC1 maturation, we found nuclear factor κB (NF-κB) and interferon (IFN) pathways to be highly enriched in a subset of functionally mature cDC1s. We identified an NF-κB-dependent and IFN-γ-regulated gene network in cDC1s, including cytokines and chemokines specialized in the recruitment and activation of cytotoxic T cells. By mapping the trajectory of intratumoral cDC1 maturation, we demonstrated the dynamic reprogramming of tumor-infiltrating cDC1s by NF-κB and IFN signaling pathways. This maturation process was perturbed by specific inactivation of either NF-κB or IFN regulatory factor 1 (IRF1) in cDC1s, resulting in impaired expression of IFN-γ-responsive genes and consequently a failure to efficiently recruit and activate antitumoral CD8+ T cells. Last, we demonstrate the relevance of these findings to patients with melanoma, showing that activation of the NF-κB/IRF1 axis in association with cDC1s is linked with improved clinical outcome. The NF-κB/IRF1 axis in cDC1s may therefore represent an important focal point for the development of new diagnostic and therapeutic approaches to improve cancer immunotherapy.
Link to Pubmed [PMID] – 34244313
Link to HAL – amu-03669200
Link to DOI – 10.1126/sciimmunol.abg3570




