Membres










À propos
Exploring the functions of the immune sentinels at the brain borders to control neuroinflammation and cognition
Background
Due to the vital importance of the brain, its development and inflammation have to be tightly controlled. While much is known about the blood-brain barrier and brain’s immune cells (microglia), less is understood about the contribution of brain borders, specifically the meninges, to brain function and inflammation. The brain is protected by the meninges, a three-layer covering that provides a structure onto which a myriad of resident innate immune sentinel cells block threatening pathogens or activate the adaptive immune system in response to inflammatory challenges. Once thought to merely shield the CNS parenchyma containing mostly neurons and glial cells, the meninges have been proven to harbor a large network of functions, at steady-state and upon inflammation.
We are using cutting-edge technologies such as intravital imaging, histo-cytometry, functional flow cytometry and single-cell transcriptomic approaches that together allow for a precise and dynamic investigation of the meninges in wildtype and transgenic mice models. We aim to unravel the remarkable complexity and plasticity of the immune sentinels’ functions in health and disease. The recent discovery of this complex and dynamic meningeal innate immunity means that the development of new targeted therapeutic agents for the treatment of neurological disorders is within the realm of possibility.
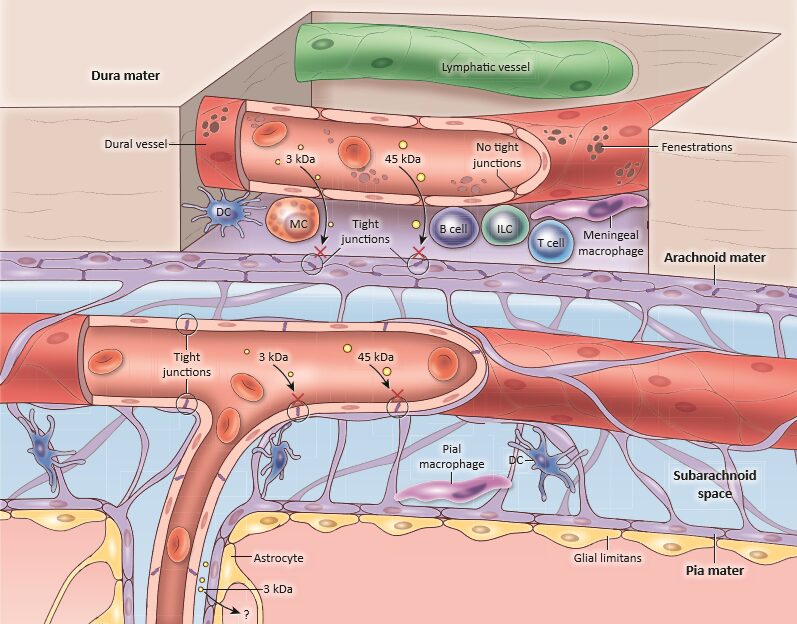
Schematic diagram of the steady-state meningeal anatomy showing the three layers (dura, arachnoid and pia) covering the brain surface. Immune composition showing the large repertoire of immune sentinels such as the Dendritic Cells (DCs), Mast Cells (MCs), Innate Lymphoid Cells (ILCs) and Meningeal Macrophages (MMs). Copyright: Rejane Rua et al., Trends in Molecular Medicine.
Previous work
Using an autoimmune disease model, we initially showed that a specific type of immune sentinels, the T-bet-dependent NKp46+ Innate Lymphoid Cells (ILCs), controlled the CNS parenchymal infiltration. These seminal findings clearly demonstrate that the meningeal immune sentinels have a key role in initiating the neuroinflammation.
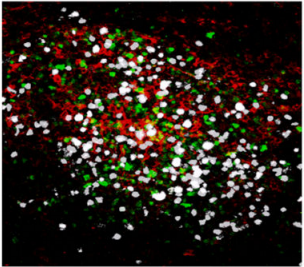
Confocal microscopy images of meningeal whole mount showing an inflammatory cluster, containing auto-reactive CD4+ T cells TH17 (white), antigen-presenting cells (red) and innate lymphocytes (green) at the peak of the EAE disease.
Copyright: Rejane Rua and Vanja Lazarevic, Nature Immunology. Artwork illustrating inflammatory clusters. Copyright: Lewis Long, Springer Nature Publishing AG.
In addition to lymphoid sentinels, the surface of the CNS is also inhabited by a vast network of myeloid sentinels. Resident Meningeal Macrophages (MMs) are the most abundant immune cells in the meninges and are constantly sampling their environment. However, little is known about how they are maintained and regulated during and after an inflammatory challenge. By following these cells upon infection by the lymphocytic choriomeningitis virus (LCMV) that causes meningitis, we showed that the pool of these specialized MMs is greatly altered, thus leading to long-term defects in their immune function.
Representative time lapses of 3D reconstructions show the dynamics of myeloid cells (green) captured by intravital imaging through a thinned skull of naive Cx3cr1-GFP/+ mice
(Top panel with video link showing steady state MMs) and through a thinned skull of LCMV-infected Cx3cr1gfp/+ mice at the peak of the disease where the LCMV-specific CTL express a fluorescent protein (pink)
(Bottom panel with video link showing interactions between MMs and CTLs at the peak of the disease). The blood vessels are labelled with Evans blue (red). Copyright: Rejane Rua et al., Nature Immunology.
Current work
Our research currently focuses on myeloid cells at the brain borders, and their roles in health and disease.
In early-life, we found that bone channels are established between the skull and the brain borders, and that they control the trafficking of myeloid cells into the meninges. Remodeling of those channels was sufficient to curb neuroinflammation. We are exploring the consequences of the modulation of this skull-to-brain axis in neonatal and adult inflammatory contexts (Eme-Scolan et al., Immunity, 2025).
During the neonatal stage, we found that meningeal macrophages support brain development, in the cerebellum and hippocampus. We are leveraging bioinformatics, genetics, and innovative drug delivery methods to unravel the underlying molecular mechanisms at play during normal development. In addition, early-life stressors are linked with neurodevelopmental disorders. We are exploring if meningeal macrophage inflammation at birth contributes to long-term cognitive disorders in mice.
In the adult stage, we found that meningeal macrophages continue to boost brain functions through alternative pathways, and are using omics-approaces to elucidate the molecular and cellular mechanisms involved in the communication between meningeal macrophages and neurons. In addition, in the context of an infection, we found that activation of meningeal macrophages can protect against fatal meningitis, acting as the first line of defense against neuroinvasive pathogens, and are exploring the underlying mechanims (Rebejac et al., Immunity, 2022).
Laslty, we are actively involved in international collaborations to understand the role of meningeal macrophages in other brain pathologies, such as meningioma and stroke.
Overall, myeloid cells at the brain borders represent new players in neuroimmunology, as they function as a communication hub between the brain and the periphery. We hope that our innovative approach to manipulate these cells will mark a significant leap forward in the fight against neurological disorders.
Actualités

À l’occasion de la Journée Internationale de l’Immunologie 2025, placée sous le thème “Brain and Immunity”, le Centre d’Immunologie de […]

Suite à l’article publié dans La Provence le mercredi 6 août 2025, les travaux de Réjane Rua, chef d’équipe Inserm […]

Réjane Rua, chercheuse à l’Inserm au Centre d’Immunologie de Marseille-Luminy (CIML – Inserm/CNRS/Aix-Marseille Université), vient d’obtenir un ERC Consolidator Grant […]
Projets
Project : UNFIRE Autres participants: Ciphe (Ana Zarubica), Hospices Civils de Lyon et CIRI (Sophie Trouillet-Assant, Thierry Walzer), équipe CoVir […]





Project : RIPIREVI Autres participants: CIPHE (Ana Zarubica et collègues). Résumé: Les interférons de type I (IFN-Is) sont essentiels à […]


Project : BAMneuro ERC Consolidator Le cerveau adulte reste extraordinairement plastique et cette adaptabilité dépend en partie des interactions continues […]

Projet: CNSentinels ERC Starting Les infections virales touchant le système nerveux peuvent provoquer des altérations majeures des fonctions cérébrales. Ce […]

INCA TUMC21-019 Le méningiome, l’une des tumeurs cérébrales les plus fréquentes, se développe au contact direct des méninges, un environnement […]

Projet: DECODIS Les méninges abritent plusieurs populations de cellules immunitaires capables d’influencer directement l’activité du cerveau, en particulier lorsqu’un événement […]

Projet: NANNYMACBRAIN Le développement du cerveau dépend d’un échange continu entre cellules immunitaires frontalières et réseaux neuronaux en formation. Nous […]