Publication: Network analysis of large-scale ImmGen and Tabula Muris datasets highlights metabolic diversity of tissue mononuclear phagocytes.
Publié dans: Cell Rep 2023 Feb; 42(2): 112046
Auteurs: Gainullina A, Mogilenko DA, Huang LH, Todorov H, Narang V, Kim KW, Yng LS, Kent A, Jia B, Seddu K, Krchma K, Wu J, Crozat K, Tomasello E, Dress R, See P, Scott C, Gibbings S, Bajpai G, Desai JV, Maier B, This S, Wang P, Aguilar SV, Poupel L, Dussaud S, Zhou TA, Angeli V, Blander JM, Choi K, Dalod M, Dzhagalov I, Gautier EL, Jakubzick C, Lavine K, Lionakis MS, Paidassi H, Sieweke MH, Ginhoux F, Guilliams M, Benoist C, Merad M, Randolph GJ, Sergushichev A, Artyomov MN
Résumé
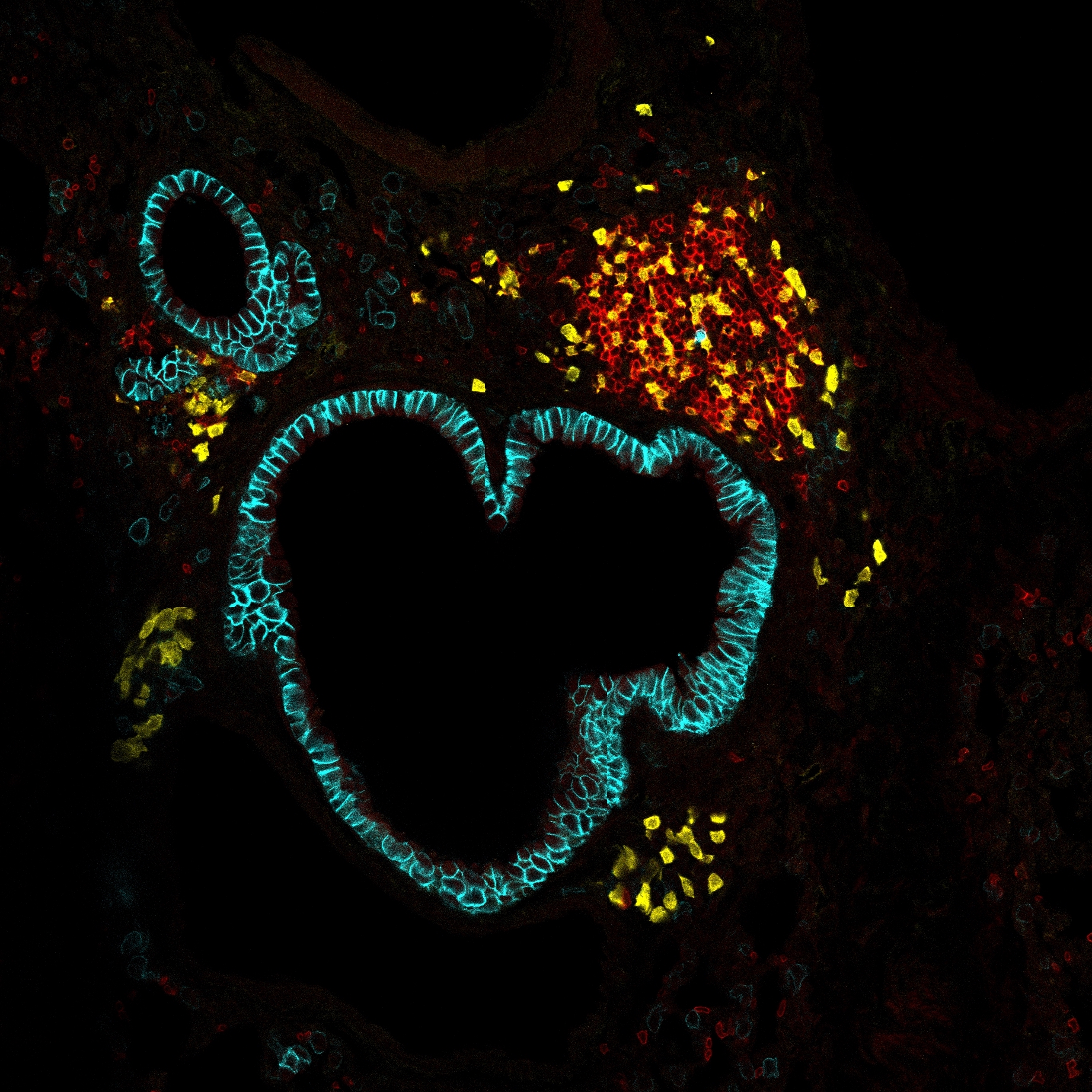
The diversity of mononuclear phagocyte (MNP) subpopulations across tissues is one of the key physiological characteristics of the immune system. Here, we focus on understanding the metabolic variability of MNPs through metabolic network analysis applied to three large-scale transcriptional datasets: we introduce (1) an ImmGen MNP open-source dataset of 337 samples across 26 tissues; (2) a myeloid subset of ImmGen Phase I dataset (202 MNP samples); and (3) a myeloid mouse single-cell RNA sequencing (scRNA-seq) dataset (51,364 cells) assembled based on Tabula Muris Senis. To analyze such large-scale datasets, we develop a network-based computational approach, genes and metabolites (GAM) clustering, for unbiased identification of the key metabolic subnetworks based on transcriptional profiles. We define 9 metabolic subnetworks that encapsulate the metabolic differences within MNP from 38 different tissues. Obtained modules reveal that cholesterol synthesis appears particularly active within the migratory dendritic cells, while glutathione synthesis is essential for cysteinyl leukotriene production by peritoneal and lung macrophages.
Lien vers Pubmed [PMID] – 36708514
Lien vers HAL – hal-04102290
Lien vers le DOI – 10.1016/j.celrep.2023.112046