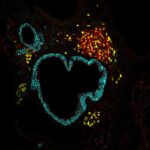
Publication: MARCH9-mediated ubiquitination regulates MHC I export from the TGN.
Publié dans: Immunol Cell Biol 2017 Oct; 95(9): 753-764
Auteurs: De Angelis Rigotti F, De Gassart A, Pforr C, Cano F, N'Guessan P, Combes A, Camossetto V, Lehner PJ, Pierre P, Gatti E
Résumé
Given the heterogeneous nature of antigens, major histocompatibility complex class I (MHC I) intracellular transport intersects with multiple degradation pathways for efficient peptide loading and presentation to cytotoxic T cells. MHC I loading with peptides in the endoplasmic reticulum (ER) is a tightly regulated process, while post-ER intracellular transport is considered to occur by default, leading to peptide-bearing MHC I delivery to the plasma membrane. We show here that MHC I traffic is submitted to a previously uncharacterized sorting step at the trans Golgi network (TGN), dependent on the ubiquitination of its cytoplasmic tail lysine residues. MHC I ubiquitination is mediated by the E3 ligase membrane-associated RING-CH 9 (MARCH9) and allows MHC I access to Syntaxin 6-positive endosomal compartments. We further show that MARCH9 can also target the human MHC I-like lipid antigen-presentation molecule CD1a. MARCH9 expression is modulated by microbial pattern exposure in dendritic cells (DCs), thus revealing the role of this ubiquitin E3 ligase in coordinating MHC I access to endosomes and DC activation for efficient antigen cross-presentation.
Lien vers Pubmed [PMID] – 28559542
Lien vers le DOI – 10.1038/icb.2017.44